Applied Chemical Engineering
|
2578-2010 (Online) Journal Abbreviation: Appl. Chem. Eng. |
Applied Chemical Engineering (ACE) is an international open-access academic journal dedicated to publishing highly professional research in all fields related to chemical engineering. All manuscripts are subjected to a rigorous double-blind peer review process, to ensure quality and originality. We are interested inthe original research discoveries. This journal also features a wide range of research in ancillary areas relevant to chemistry. ACE publishes original research articles, review articles, editorials, case reports, letters, brief commentaries, perspectives, methods, etc. The research topics of ACE include but are not limited to:
Starting from Volume 7, Issue 2 of 2024, Applied Chemical Engineering (ACE) will be published by Arts and Science Press Pte. Ltd. Please turn to the journal website for new submissions. |
Vol. 9 No. 1 (2026): Publishing
Table of Contents
Announcements
This journal will be jointly published by Enpress Publisher and Arts and Science Press (https://ojs.as-pub.com/index.php/index/index). |
|
| This journal will be jointly published by Enpress Publisher and Arts and Science Press (https://ojs.as-pub.com/index.php/index/index). | |
| Posted: 2024-01-25 | |
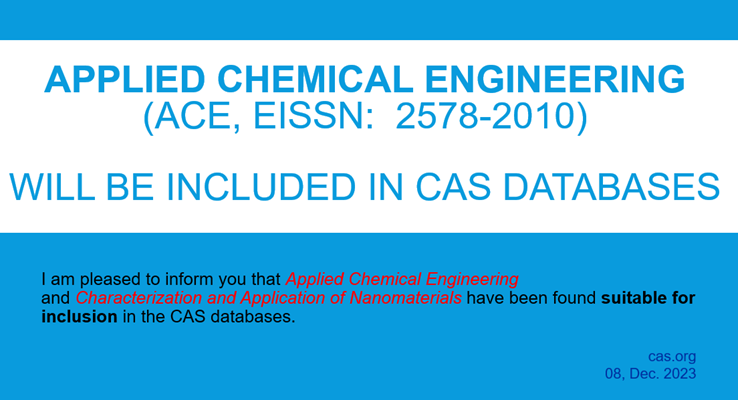
ACE is included in CAS databases! |
|
 |
|
| Posted: 2023-12-11 | |
Publication frequency becomes quarterly |
|
 |
|
| Posted: 2023-09-12 | |
| More Announcements... |









 ISSN:
ISSN:  Open Access
Open Access